Unique RFID product with "minimum size"
Tageos, a member of AIPIA, which provides RFID inlays and tags, has launched the eos-202u9 RAIN RFID inlay. This new inlay has successfully passed the tests specified by the Auburn University RFID laboratory specifications and passed the DoseID certification, which is suitable for a wide range of pharmaceutical and medical applications.
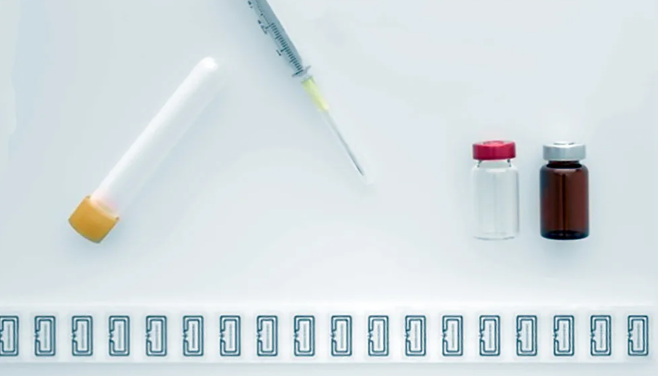
DoseID is an industry consortium that uses RFID technology in the healthcare and pharmaceutical fields to authenticate RFID inlays used to track medicines. The COVID-19 pandemic has highlighted the global demand for high-performance, flexible medical solutions. RFID technology has made a great contribution in this regard by seamlessly tracking and tracing medicines on the basis of the user unit.
They are fundamentally upgrading their mosaic series and launching a product with unique features. The new UHF product uses a sophisticated, very small antenna design, measuring only 20x10 mm (wet inlaid finished product size 22x12 mm), using NXP's most advanced UCODE9 IC and unique brand identifier and pre-serialization 96-bit EPC memory.
According to Tageos, the chip and antenna design is customized for eos-202u9, which is the smallest RAIN RFID inlay on the market. According to the ARC Spec S standard for healthcare and pharmaceutical applications, eos-202u9 is currently tested in all types The project has shown strong performance.
Its sensitivity and reliability, even in the dense population of RFID tags, enable inlays to be used in the latest test item portfolio outlined by ARC Spec S, including clear and amber liquid glass bottles, clear glass powder bottles, plastic/COC syringes, Plastic syringe caps and plastic blow-fill sealed bottles. It does not have any size disadvantages, compared to other existing certified inlays, it claims. The new inlay can be used immediately in dry, wet, and paper formats.
Chris Reese, head of product management at Tageos, said: "Due to its unique combination of minimum size and optimal reading characteristics in challenging environments, the new inlay provides the ultimate'one size fits all' solution for the healthcare and pharmaceutical industries."
Kevin MacDonald, the co-founder and CEO of Kit Check, is a founding member of DoseID and a provider of automated drug tracking and transfer detection solutions for American hospital pharmacies. He commented: "It looks like eos-202u9 has achieved'dominance of the world'. Promise-this is good news for us and all our customers."
They are fundamentally upgrading their mosaic series and launching a product with unique features. The new UHF product uses a sophisticated, very small antenna design, measuring only 20x10 mm (wet inlaid finished product size 22x12 mm), using NXP's most advanced UCODE9 IC and unique brand identifier and pre-serialization 96-bit EPC memory.
According to Tageos, the chip and antenna design is customized for eos-202u9, which is the smallest RAIN RFID inlay on the market. According to the ARC Spec S standard for healthcare and pharmaceutical applications, eos-202u9 is currently tested in all types The project has shown strong performance.
Its sensitivity and reliability, even in the dense population of RFID tags, enable inlays to be used in the latest test item portfolio outlined by ARC Spec S, including clear and amber liquid glass bottles, clear glass powder bottles, plastic/COC syringes, Plastic syringe caps and plastic blow-fill sealed bottles. It does not have any size disadvantages, compared to other existing certified inlays, it claims. The new inlay can be used immediately in dry, wet, and paper formats.
Chris Reese, head of product management at Tageos, said: "Due to its unique combination of minimum size and optimal reading characteristics in challenging environments, the new inlay provides the ultimate'one size fits all' solution for the healthcare and pharmaceutical industries."
Kevin MacDonald, the co-founder and CEO of Kit Check, is a founding member of DoseID and a provider of automated drug tracking and transfer detection solutions for American hospital pharmacies. He commented: "It looks like eos-202u9 has achieved'dominance of the world'. Promise-this is good news for us and all our customers."